All published articles of this journal are available on ScienceDirect.
The Use of Higher Secretory Immune Response of IgG And IgA in Breast Milk and Blood as a Donor Therapy in COVID-19 Survivor Mothers
Abstract
Introduction:
The breast milk of mothers recovering from COVID-19 has elevated levels of secretory-type antibodies, such as IgG and IgA. Furthermore, these antibodies serve as immunity, which can be passed on to the baby through breastfeeding.
Objective:
This study aimed to analyze the relationship between IgG and IgA levels in breast milk and blood of breastfeeding mothers who recovered from COVID-19 and assess other determinants.
Methods:
This was an analytical study, which was carried out using 54 participants who were equally divided into two groups, namely case and control. The case group consisted of individuals who were exposed to COVID-19, while the controls were not exposed. Blood and breast milk (each 5 CC) were then collected to determine the levels of IgG and IgA using the Eliza method. Furthermore, the test used 27 blood and 18 breast milk samples.
Results:
The statistical analysis showed that there were significant differences in the levels of IgG and IgA in the breast milk and blood of the case and control groups. The average IgA in the blood and breast milk of the control was greater compared to the case group. Furthermore, the average IgG in the breast milk of the case group was greater compared to the controls. Based on the results, there was no difference in mean IgG in breast milk in both groups.
Conclusion:
The results showed that there were differences in mean IgA in the breast milk of both groups. The average IgA in the blood of the controls was greater than the case group, but the IgG in breast milk was lower.
1. INTRODUCTION
Coronavirus disease 2019 (COVID-19) is a global health threat, primarily transmitted through respiratory droplets and aerosol transmission, presenting significant and pressing challenges [1]. Furthermore, a previous report showed that the number of confirmed positive cases around the world was 162 million as of May 15, 2021 [2]. In the context of COVID-19 incidence, the Province of East Kalimantan ranked fifth in Indonesia, with a total number of 1,734,285 cases.
Histopathological and ultrastructural examinations have provided compelling evidence regarding the presence of SARS-CoV-2 particles within placental tissues, suggesting a potential impairment of the fetal-maternal barrier [3]. During the COVID-19 pandemic, there is a risk that mothers can transmit SARS-CoV-2 to their babies or children while breastfeeding. Several studies have also reported that the breast milk of mothers exposed to COVID-19 contains immunity with the ability to protect babies from the virus. However, there is no evidence indicating the presence of SARS-CoV-2 in the breast milk of infected parents.
One of the recommended measures for breastfeeding mothers is to maintain respiratory hygiene, specifically during feeding sessions. The use of a face mask is advised around a breastfed in cases of shortness of breath. Furthermore, the hands should be washed thoroughly with soap or sanitizer for a least 20 seconds before and after touching the baby. Any surfaces to be touched must also be routinely cleaned and disinfected. In cases where a mother is severely ill with COVID-19, is suspected of having the virus, or experiences other complications serving as an obstacle to breastfeeding, expressing milk safely is crucial [4]. The use of relactation (restarting breastfeeding after a gap) or donor human milk from certified banks can be used in critical maternal cases. According to previous studies, the adopted approach is majorly influenced by cultural context, patient acceptability, and availability of services.
Mothers and infants are advised to stay together, practice skin-to-skin contact, and room-in throughout the day and night, regardless of their COVID-19 status. This is very crucial immediately after birth during the establishment of breastfeeding. Several studies have shown that children are all susceptible to COVID-19, irrespective of their gender [5].
During the COVID-19 pandemic, there were concerns regarding the potential transmission of SARS-CoV-2 from mothers to their babies or children. Recommendations concerning mother-infant contact and breastfeeding should be based on several factors, including the absence of infection risk to infants, morbidity, mortality due to breastfeeding, inappropriate milk formula milk, and the protective effect of skin-to-skin contact. WHO has recommended that mothers with COVID-19 must continue providing milk, keep rooming in, engage in skin contact, and initiate early breastfeeding immediately after birth. Counseling must also be provided to increase confidence and understand the benefits of breastfeeding [2].
Breast milk is rich in secretory-type antibodies, such as Immunoglobulin G (IgG) and Ig A, particularly in mothers recovering from COVID-19. Furthermore, it contains immune substances, which act as a protector for babies who are passively immunized [6]. In mothers exposed to COVID-19, it contains immunity that protects babies from the virus [6, 7] Breastfeeding mothers suffering from SARS-CoV-2 have been reported to have increased levels of T-cells enriched with functional mucosal memory for infant protection [8]. Based on findings, there was no evidence of SARS-CoV-2 RNA in the breast milk of individuals with SARS-CoV-2. All breast milk samples contain IgG and IgA, and there was a correlation between the levels of coronavirus and SARS-CoV-2 immunity [9].
Several studies have reported the prevalence of SARS-CoV-2 IgA and IgG in mothers who recovered from exposure to COVID-19, with this immunity lasting for up to 8 months. This condition holds the potential to protect breastfed babies against the risk of transmission and severity of the disease [6]. In the ongoing effort to combat the worldwide outbreak [6] of COVID-19, it is imperative to investigate factors, such as specific antibodies present in breast milk, that can provide immunity, particularly for vulnerable newborns. Breast milk is rich in secretory-type antibodies (sIgG), which can be transferred to infants through breastfeeding [10]. According to previous reports, purified antibodies also have therapeutic effect on affected adults [7]. Therefore, this study aimed to analyze the relationship between IgG and IgA levels in breast milk and blood of breastfeeding mothers who have recovered from COVID-19, as well as assess the adherence to breastfeeding protocols and other determinants. During the COVID-19 pandemic, several mothers lost the opportunity to breastfeed due to separation from their babies, leading to negative impacts [11]. The risk of postpartum Post-Traumatic Stress Disorder (PSTD) can affect formula-fed infants, rooming in, skin-to-skin contact, and early initiation of breastfeeding. In these conditions, healthcare professionals need to provide support to prevent the occurrence of PSTD [12]. The incidence of COVID-19 led to the implementation of certain policies that prevented mothers from accessing their babies, leading to the cessation of breastfeeding. This indicates that professionals need to provide support to facilitate the continuous provision of breast milk to increase immunity in infants, thereby preventing such incidents in the future [11].
2. STUDY METHOD
2.1. Method
This was an analytical study on breastfeeding mothers who were exposed to COVID-19. A total of 5 CC of blood and breast milk were taken to assess the IgG and IgA levels, followed by collection of data on the characteristics of the mother and baby. The examination for IgG, IgA, and Anti-SARS-CoV-2 antibodies utilized reagents from RayBiotech, identified by LOT numbers 062429005 for IgG and 0624229101 for IgA. The assessment employed the ELISA (Enzyme-Linked Immunosorbent Assay) technique, specifically the indirect method. This ELISA procedure was conducted at the Tropical Disease Center Laboratory, Universitas Airlangga. The blood and breast milk samples were examined for IgG, IgA, and Anti-SARS-CoV-2 to determine whether they contained antibodies against COVID-19. The sample population consisted of 54 participants who were evenly divided into 2 groups, namely case and control, as well as selected using the purposive sampling techniques. The inclusion criteria included mothers whose children were alive and willing to provide blood and breast milk, which could limit the generalizability of the findings. The data collected were analyzed using univariate analysis with percentages to determine the differences in blood and breast milk IgG levels between COVID-19 mothers and controls, followed by bivariate analysis with T-test. This study was conducted from May to July 2022, with an ethical test number: L.B.02.01/7.1/06737/2022. The procedures were approved by the Health Research Ethics Committee of the Health Polytechnic of the Ministry of Health, East Kalimantan, Indonesia.
| No. | Characteristics | Case | Control | ||
|---|---|---|---|---|---|
| (n) | (%) | (n) | (%) | ||
| 1 | Aged < 20 age 20-35 age > 35 age |
0 20 7 |
0 72,0 28,0 |
0 20 7 |
0 72,0 28,0 |
| 2. | Education Primary school Junior high school Upper middle school College |
7 3 7 10 |
28,0 12,0 28,0 37,0 |
4 5 13 5 |
15,0 19.0 48,0 19,0 |
| 3. | Paritas Primipartias Multiparitas Grandemulti |
8 16 3 |
32,0 56,0 12,0 |
8 15 4 |
32,0 55.0 15.0 |
| 4. | Gestational Age 31-36 weeks 37-40 weeks 41-42 weeks |
4 20 3 |
15,0 74,0 11,0 |
0 27 0 |
0 100.0 0 |
| 5. | Types of Childbirth Spontaneous Sectio Caesar |
9 18 |
36,0 64,0 |
16 11 |
56,0 44,0 |
| 6 | Birth Weight Usual (2500 – 4000) Premature (< 2500) |
21 6 |
78,0 22,0 |
25 2 |
93,0 7,0 |
| 7 | Covid Vaccine (2x) Yes Not |
11 16 |
44,0 36,0 |
15 12 |
56,0 44,0 |
| 8 | Hospital Duration 0 1 - 2 Day 3- 4 Day 5- 6 Day 7- 8 Day 9- 10 Day 11- 12 Day 13 -14 Day 15 – 16 Day 17 -18 Day 19 -20 Day >20 Day |
- 15 5 1 3 - 1 1 - - - 1 |
- 55,0 19,0 4,0 12,0 - 4,0 4,0 - - - 4,0 |
- 25 2 - - - - - - - - - |
- 93,0 7,0 - - - - - - - - - |
| Total | 27 | 100 | 27 | 100 | |
3. RESULTS AND DISCUSSION
According to Table 1 on the case respondents, the majority were aged 20-35 years, totaling 18 individuals (72%), Furthermore, 8 of them graduated from college (37%), 16 had multiparity (56%), 20 had a gestational age of 36-39 weeks (60%), 18 experienced SC deliveries (64%), and 21 had infants with normal birth weights (78%). The results showed that 11 (44%) and 15 (55%) participants had been vaccinated twice in the case and control groups, respectively.
Based on Tables 2, 3, and 6, babies were born with LBW in the case and control groups, respectively. Furthermore, 4 babies were treated in the NICU, and 1 had umbilical cord entanglement in the case group. Among the case and controls, 1 participant experienced shortness of breath.
Table 4 showed that among the 27 respondents exposed to covid 19 whose breast milk was produced, 18 of them had minimum blood IgG level of 1131 IU, with a maximum and mean of 105774 IU and 40440, respectively. Furthermore, the minimum ASI IgG level was 4405 IU, with a maximum and mean of 289530 and 146174, respectively. The ASI Sarcov level and the blood of all respondents were negative, assuming the values were below 1. The ASI IgG levels were 3.6 times higher compared to the IgG levels.
Among the control group, the minimum blood IgG level was 6200.71 IU, with a maximum and mean of 95616.86 IU and 40440.80, respectively. The minimum ASI IgG level was 21638.73 IU with a maximum and mean of 236573.7 and 120167.044, respectively. The breast milk and blood Sarcov levels of all respondents were negative, assuming the value was below 1. Furthermore, breast milk IgG levels were 2.3 times higher compared to the blood IgG levels.
| No. | Characteristics | (n) | (%) | (n) | (%) |
|---|---|---|---|---|---|
| Case | Control | ||||
| 1 | Day Milk Expenditure 1-2 Have Nothing |
13 14 |
48,0 52,0 |
19 8 |
70,0 30,0 |
| 2 | Milk ejection during sampling Have Nothing |
18 7 |
64,0 36,0 |
27 0 |
100,0 0 |
| 3 | Formula milk provider Have Nothing |
16 11 |
56,0 44,0 |
1 26 |
4.0 96,0 |
| 4 | Breastfeeding mothers Have Nothing |
18 7 |
64,0 36,0 |
26 1 |
96,0 4,0 |
| No. | Problem | Case | Control |
|---|---|---|---|
| 1 | Prematur (BB <2500 Grm) | 6 | 2 |
| 2 | NICU admitted | 4 | - |
| 3 | Umbilical Cord Winding | 1 | - |
| 4 | Crowded | 1 | 1 |
| - | N | Min. | Maximum | Mean | Std. Deviation | N | Minimum | Maximum | Mean | Std. Deviation |
|---|---|---|---|---|---|---|---|---|---|---|
| Case | control | |||||||||
| IgG blood | 27 | 1131.96 | 105774.58 | 40440.80 | 33454.78 | 27 | 6200.71 | 95616.86 | 52252.18 | 28111.50 |
| Sarcov Darah | 27 | 0 | 0.508 | 0.10 | 0.14 | 27 | .01 | .23 | .13 | .085 |
| IgG Breast milk | 18 | 4405.16 | 289530.01 | 146174.37 | 97794.70 | 27 | 21638.73 | 236573.75 | 120167.04 | 55480.72 |
| Sarcov breast milk | 18 | 0.123 | 0.644 | 0.33 | 0.15 | 27 | .20 | .48 | .35 | .11 |
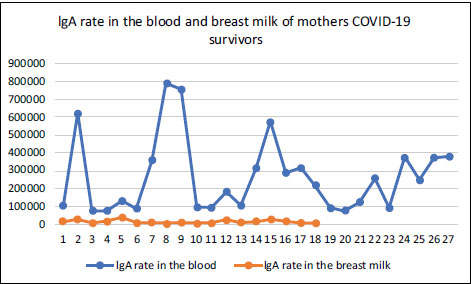
Based on Table 5, the average IgA in the Control blood was greater compared to the case group. Furthermore, there were differences in the mean IgA in breast milk of the cases and controls, with the Whitney test showing a value of 0.000. The average IgA in the breast milk of the case group was lower compared to the controls. The results showed that there was no difference in the mean IgA of the case and control groups, with the Whitney test showing a value of 0.539 (Grafik 1).
The average IgG in the control blood was greater compared to the case group. Furthermore, there was no difference in the mean IgG between both groups. The Independent T-test showed a p-value of 0.152.
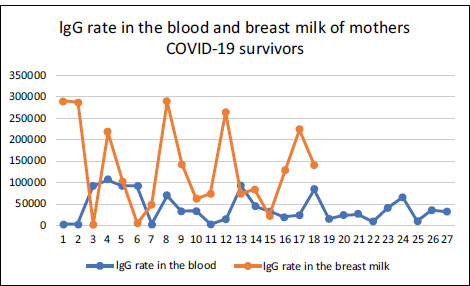
The average IgG in breast milk of the case group was greater compared to the controls. Based on the results, there was no difference in mean IgG in the breast milk of the case and control groups. The independent T-test results showed a p-value of 0.505 (Grafik 2).
| Distribution of Ig A | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| - | - | Case | Control | ||||||||
| - | N | Mean | Min. | Max. | SD | Mean | Min. | Max. | SD | P-value | |
| Ig A blood | 27 | 266542 | 74046 | 791326 | 210342 | 184173 | 75333 | 384395 | 78400 | 0,539 | |
| Ig A breast milk | 18 | 11826.6 | 1946.7 | 36267.9 | 10049.3 | 183526 | 613 | 289530 | 101384 | 0,000 | |
| - | - | Distribution of Ig G | - | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| - | - | Case | Control | - | ||||||
| - | N | Mean | Min. | Max. | Sd | Mean | Min. | Max. | SD | P-value |
| IgG blood | 27 | 184173 | 1132 | 105775 | 33455 | 83337 | 1132 | 105775 | 33455 | 0,152 |
| IgG breast milk | 18 | 135697 | 613 | 289530 | 101384 | 119170 | 7506 | 641947 | 173386 | 0,505 |
| No. | Variable | P-value | α | Variable | P-value | α |
|---|---|---|---|---|---|---|
| Case | Control | |||||
| 1 2 |
IgG blood – breast milk IgA blood – breast milk |
0.000 0,569 |
0.05 0.05 |
IgG blood – breast milk IgA blood – breast milk |
0,565 0, 455 |
0,05 |
| 3 | Sarcov blood – breast milk | 0.470 | 0.05 | Sarcov blood – breast milk | 0,099 | 0,05 |
Product Moment correlation analysis for COVID-19 survivors showed that there was a relationship between the blood IgG and ASI IgG in breastfeeding mothers, with a P value of 0.000 α 0.05. However, there was no relationship between blood IgG and ASI Sarcov level, with a p-value of 0.470 α 0.05.
The Product Moment correlation analysis for non-COVID-19 survivors showed no relationship between blood IgG and ASI IgG in breastfeeding mothers suffering from COVID, with a P value of 0.565 α 0.05. Based on the results, there was no association between blood IgG and ASI Sarcov levels, with a p-value of 0.099 α 0.05.
4. DISCUSSION
In this study, a total of 27 mothers who had been exposed to COVID-19 and another 27 who were not exposed to the virus served as participants. Among the exposed group, 18 of them were actively breastfeeding and provided breast milk samples. From the results of blood and breast milk tests in the participants, SARS-Cov2 levels with the Eliza method in cases and controls were less than 1 IU. This showed that there was no transmission of COVID-19 both through blood and breast milk to the baby. Most of the participants were aged 20-35 years, with the majority of them being in college education (Case) and high school (Control).
The age range of 20-35 years was the safe age to carry out reproductive functions of pregnancy, childbirth, and breastfeeding. Several education studies on mothers who were exposed to COVID-19 identified 10 individuals (37%) in the university setting. This indicated that highly educated mothers had the willingness to use health facilities to facilitate diagnosis and the treatment of various health problems.
WHO stated that breast milk was an ideal food for babies because it was safe, clean, and provided all the energy and nutrients needed by babies for the first months of life. Breast milk contained antibodies, protecting children against several childhood diseases, and breastfeedingprovides various short- and long-term health benefits for both mother and child. Based on this finding, efforts must made to provide appropriate advice and support to the mother, even in cases of confirmed or suspected COVID-19 infection [5, 13].
Policymakers and practitioners learned from the HIV pandemic and avoided undermining breastfeeding during the COVID-19 pandemic. It was evident that to optimize infant health and well-being, COVID-19 policies must promote skin-to-skin contact, maternal proximity, and breastfeeding [14]. A total of 22% of babies in this had low birth weight and were born in premature conditions.
Premature babies were treated in the neonatal intensive care unit (NICU), with no rooming in and breastfeeding was suboptimal. This was consistent with recent studies from the World Health Organization (WHO) and its partners, stating that the COVID-19 pandemic caused a significant decline in the quality of care provided to premature and ill newborns, leading to avoidable suffering and fatalities. Meanwhile, the impact of the non-availability of breast milk and separation between mother and baby could be significant. Based on these findings, it appeared that COVID-19 in infants and children posed a much lower threat to survival and health compared to other infections protected by breastfeeding. Adherence to infection prevention and control measures was critical to prevent contact transmission between mothers with suspected or confirmed COVID-19 and their newborn and premature infants [2].
In conditions of low birth weight, prematurely, coolie contact with the skin of the mother and baby was very necessary. However, during the COVID-19 pandemic, mothers and babies were separated, which had an impact on the risk of death in infants [2].
This study also showed that there was no transmission of COVID-19 through breast milk or blood. There was no sufficient data to infer vertical transmission of COVID-19 from breastfeeding. Furthermore, breastfeeding newborns of mothers infected with COVID-19 was safe. Adequate infection control measure to avoid mother-infant transmission was by giving pasteurization of donor breast milk until the exclusive duration was achieved (Pereira et al., 2020). Due to the possibility of transmission from mother to baby, it was necessary to consider certain characteristics with the potential to spread the disease [15]. Strategies should be implemented to prevent mother-to-baby exposure by close prenatal, natal, and postpartum management [15].
Based on previous findings, it could be concluded that all the options regarding breastfeeding were justifiable due to the novelty of the infection. However, puerperal women and their families should be very well informed to make a conscious choice in line with the aforementioned information.
The World Health Organization (WHO) recommendations for the early initiation of breastfeeding and its continuation for infants and young children also extended to mothers who were suspected or confirmed to have COVID-19. This was due to the presence of IgA in breast milk, which provided a protective mechanism against infection and death. Although IgA antibodies with reactivity to the SARS-CoV-2 virus had been detected in the breast milk of mothers who had recovered from the infection, there had been limited studies on their strength and durability to protect against COVID-19 in breastfed infants.
Studies have shown that beyond the neonatal period, the benefits of mother-infant physical contact included improved sleep patterns, reduced behavior issues in children, and increased quality of interaction with parents [17]. Furthermore, there was an ongoing discussion among the scientific community to determine the most effective methods for promoting and implementing breastfeeding, while also adhering to protocols designed to prevent neonatal infections during the COVID-19 pandemic [17]. Breastfeeding also provides various health benefits for the mother, including protection against breast cancer, ovarian cancer, and type 2 diabetes [19].
Studies have shown that breast milk contains high levels of secretory immunoglobulin A (sIgA) antibodies. A mother who recovered from COVID-19 could pass on immunity to the baby through breastfeeding. Furthermore, purified breast milk antibodies could be used as a therapeutic option for adults with the virus [19]. The presence of sIgA antibodies could help the immune system recognize and combat pathogens, including COVID-19. All mothers who had recovered from COVID-19 had antibodies in their breast milk, providing opportunities to use this as a treatment for critically ill infants or to prevent severe illness in susceptible infants. Breast milk also contained high levels of IgA antibodies, which were particularly effective against diseases attacking the respiratory tract, such as COVID-19 [7].
All breastfeeding mothers who had received the Sars-Cov-2 vaccine possessed IgG antibodies. The average IgG antibody content in these individuals was 3379.6 Â ± 1639.5 /mL. The results showed that 89% of the breastfeeding mother's milk samples contained IgA. The number of antibodies in the breast milk of mothers who were breastfeeding for 24 months was significantly higher compared to those with a lesser duration (0.001) [21]. This indicated that the milk contained innate immunological properties with the ability to resist COVID-19 [2, 18, 19]. Further studies must compare the potency of the antibodies present in breast milk to those present in the blood. The levels of sIgA in breast milk surpassed those found in plasma, and sIgA provided substantial protection for the respiratory tract [7, 20].
Breast milk was widely recognized as the optimal form of nutrition for infants and provided a range of protective benefits against various diseases. However, there were some rare exceptions where breastfeeding or offering expressed breast milk could not be recommended. Mothers who were not infected with COVID-19 or had not been in close contact with a confirmed case did not require additional precautions while providing breast milk. However, it was important for all mothers engaging in breastfeeding and using breast pumps to have a proper understanding of how to clean and sanitize the equipment to ensure safe and hygienic use [2].
Early breastfeeding initiation (EBI) was carried out based on a joint decision with parents. Information about the risks and benefits of early breastfeeding was provided, as well as the mechanism of COVID-19 virus transmission. Early initiation was carried out if the status of the mother was in close contact or a suspected case. EBI could also be considered in mothers with confirmed status (mild symptomatic/asymptomatic), and the mother and newborn had both been clinically declared stable. The protocol for preventing COVID-19 transmission must be carried out through the use of PPE or at least a mask [22, 23]. Policymakers and practitioners must also learn from the mistakes of the HIV pandemic and not hinder the provision of breastmilk during the COVID-19 pandemic. To maximize infant health and well-being, COVID-19 policies were expected to support skin-to-skin contact, maternal closeness, and breastfeeding.
The Indonesian Pediatricians Association stated that there were three options for the provision of nutrition to babies born to mothers with suspected and confirmed COVID-19 status, depending on the clinical condition of the mother. In critical cases, the baby was given donor breast milk or formula milk. In moderate cases, pumped breast milk was provided, while direct breastfeeding was allowed in mild cases with strict adherence to guidelines. The mother was advised to wear a mask when breastfeeding and caring for the baby, wash her hands with soap and running water before and after the process and spray disinfectant on all surfaces and items used [22]. In a cohort study of six breastfeeding women who received two doses of the SARS-CoV-2 vaccine, there was a significant increase in the levels of SARS-CoV-2 specific IgG and IgA antibodies in breast milk, starting on the 7th day after the initial vaccine dose, with a predominant response of the IgG [23].
The humoral immune response was usually characterized by a primary IgM antibody response, followed by a secondary antibody response associated with immune system memory of IgG, IgA, and IgE. Furthermore, IgA plays a crucial role in protecting mucosal surfaces against pathogens by neutralizing viruses or by inhibiting viruses from adhering to the body's barrier epithelial cells. Immunoglobulin A was effective in preventing infection in mice and humans compared to influenza-specific IgG, and increased serum IgA levels correlated with the efficacy of influenza vaccination. IgA played an important role in SARS-CoV infection. Mice given an intranasal vaccine with the SARS-CoV protein induced a specific IgA response and this protein provided better protection against SARS-CoV compared to intracellular administration of the vaccine [22, 24]. Breastfeeding reduces the risk of both short-term and long-term infectious and non-infectious conditions. Breast milk contains immunoglobulins and bioactive factors from the response to a viral infection from the mother. The constituent IgA could inhibit the virus from entering through the epithelial barrier in the mucosa of infants or children [21].
The COVID-19 pandemic significantly impacted the global operations of human milk banks. This indicated that several measures should be taken to reduce the negative impact on the health and well-being of vulnerable newborns. Furthermore, breast milk provides vital benefits for newborns, including the possibility of transferring disease-fighting antibodies and long-term advantages. The primary goal of human milk banks globally was to promote and enhance breastfeeding. These organizations in developing countries had a crucial role to play during challenging times, providing support for the most vulnerable preterm infants by facilitating access to breast milk and supplying donated samples [7].
This study showed that a strong response to SARS-CoV-2 antibodies, dominated by sIgA, was likely to occur in a considerable proportion of individuals after infection. Further studies are needed to determine the role of these antibodies and the potential use of sIgA extracted from milk for therapeutic purposes [9]. The findings showed that the average Ig G in breast milk of the case group was higher compared to the controls.
CONCLUSION
In conclusion, the findings of this study showed that:
1. There was a relationship between blood IgG and breast milk IgG in breastfeeding mothers who were exposed to COVID-19.
2. The IgG in the breast milk of mothers exposed to COVID-19 was 3.6 times higher compared to that of the blood, while the control group was 2.3 times higher. This indicates that the breast milk of mothers exposed to COVID-19 had the opportunity to be utilized as donor breast milk.
3. There were differences in mean IgA in breast milk of the case and control groups, with a Whitney test result of 0,000. The average IgA in breast milk of the case group was lower compared to the controls.
4. WHO protocols for the care of mothers who were exposed to COVID-19 had not been implemented, where separation still occurred and some children did not breastfeed.
5. The transmission of COVID-19 from the diet and breast milk of breastfeeding mothers who were exposed to the virus had not been proven. Lab results showed that Sarcov values in both blood and breast milk were below 1 IU with the ELIZA test.
This study had several limitations, including the limited sample, which limited the statistical power of the analysis. The purposive sampling method was utilized and this could limit the generalizability of the findings. Furthermore, the participants were all from Samarinda, Indonesia, and the findings could not be generalized to other locations or cultures. Differences in educational levels between the COVID-19 and control groups could have introduced confounding variables. Future studies were advised to consider these limitations and aim for larger and more diverse sample sizes.
FUTURE STUDY
Future studies could explore the differences in breastfeeding recommendations for mothers who had recovered from COVID-19 and those who had not. Furthermore, the correlation between the levels of IgG in breast milk and blood necessitated further investigation better to understand the immunity transfer from mother to infant.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was conducted with an ethical test number L.B.02.01/7.1/06737/2022 by the Health Research Ethics Committee of the Health Polytechnic of the Ministry of Health, East Kalimantan, Indonesia.
HUMAN AND ANIMAL RIGHTS
No animals were used that are the basis of this study. Al the human experiments were performed as per the guidelines described in Helsinki Declarations.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are available from the corresponding reasonable request [E.W.W].
FUNDING
This study was funded by the Ministry of Health through the Simlitabmas grant.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGEMENTS
The authors are grateful to the Ministry of Health for funding this study, the Director of the Health Polytechnic for granting permission for the procedures, and the Director of the Tropical Disease Hospital, Surabaya, as the site for testing blood and breast milk samples.


